The Marcotte lab is developing novel systems-biology approaches to mine the evolutionary relationships between genes. These methods have been used to predict surprising relationships between genes and human disease, including:
- Yeast genes relevant for blood vessel formation in humans
- Plant genes involved in human birth defects
- Worm genes relevant to breast cancer
- Mouse genes relevant to autism
Finding disease genes from lower organisms-
New algorithms (the phenolog method, PNAS, 2010) have helped us identify non-obvious models of human diseases, including a yeast model for angiogenesis defects, a worm model for breast cancer, and a plant model for craniofacial alterations and deafness. Illustration by Katherine Weir. Vector female silhouette under Creative Commons Attribution 2.0 from ‘Keep Fit’ Vector Pack, Blog.SpoonGraphics.
We’ve now experimentally validated >100 candidates for diverse traits in a wide range of organisms, including yeast, C. elegans, Arabidopsis, frogs, mice, and humans. In our own lab, we primarily culture yeast, C. elegans, and mammalian cells; we also collaborate extensively with experts on other model systems, allowing us to test the generality of our methods. For example, in yeast we’ve predicted and validated many new ribosome biogenesis genes. In C. elegans, we predicted suppressors of the loss of the Retinoblastoma tumor suppressor, ‘curing’ worms of model tumors. In Arabidopsis, we’ve rationally identifi�ed genes regulating root growth, drought resistance, and pigmentation. In vertebrates, we’ve used gene networks to assign functions to a birth defect gene and to identify entirely new birth defect genes, confirming their roles in vivo.
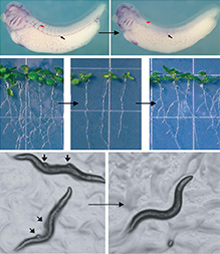
Examples of validated computational predictions.
In Xenopus, a new regulator of angiogenesis (PNAS, 2010); Arabidopsis, a new regulator of lateral root formation (Nature Biotech, 2010); and C. elegans, a new regulator of the Retinoblastoma tumor suppressor (Nature Genetics, 2008).


